In this section I'll give you my version of how the greenhouse effect works and how the model applies to the observed buildup of carbon dioxide (CO2) in Earth's atmosphere that is currently warming the planet.
It's hot in a greenhouse

If you've ever been inside a greenhouse on a sunny day, you know it can be very hot. It's a fact you can check for yourself, or go ask someone at your local plant nursery. In fact, because the plants in a greenhouse are usually somebody's investment, manufacturers work hard to keep the interior temperature from getting so high that it could kill those plants.
One common method of releasing built-up heat, an automatic window opener, is shown here. It's an ingenious device, and nearly fool-proof. A cylinder containing a fluid that expands when it heats up pushes on a piston that is connected to the window-opening mechanism. As the greenhouse warms, so does the fluid. At some point, the expanding fluid pushes the piston, opening the window, which cools the house. No power is required, just springs and expanding fluid. So as long as an opener like this is in good working order, hot air can escape and the plants are OK. Science!
Digression: The electromagnetic spectrum
In order to understand the rest of this discussion, you'll need to know a little bit about light and the electromagnetic spectrum. Here are the basics.
Light (electromagnetic radiation) is energy moving through space and objects (like glass). In science, when we say "light", we don't just mean the colors of the spectrum, ROYGBIV. We mean any electromagnetic wave, characterized by a wavelength and a frequency, and traveling at up to the speed of light, about 3 × 108 (300,000,000) m/s, or 286,000 miles/second. The electromagnetic spectrum is shown below. We humans have named certain regions to help chunk it up a bit, but there's nothing essentially different between radio waves and x-rays, except that the latter has a shorter wavelength, a higher frequency, and carries more energy.
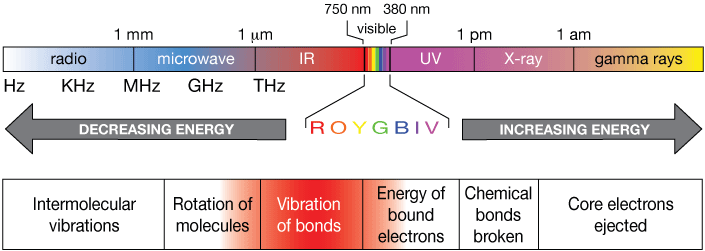
Now let's take a look at some of the key regions of the spectrum and see how they affect matter.
Radio and microwave
In these regions of the spectrum, the weak bonds between molecules in liquids and solids are excited (stimulated to vibrate more rapidly), and molecules are caused to rotate more rapidly. Microwave ovens irradiate* food with microwaves, which causes the water molecules present in all foods to rotate faster. That rapid rotation is eventually converted to vibrations of the bonds of water molecules, which we know as heat.
Infrared
Light of the energy range we call infrared (IR) is capable of stimulating a whole range of vibrations of bonds in molecules, like the double bond between carbon and oxygen in O=C=O (carbon dioxide). It's these vibrational motions we commonly experience as heat. In our atmosphere, IR light from the sun causes more rapid vibration of the bonds of carbon dioxide (CO2), water (H2O), methane (CH4) and other small gases present there. So these molecules are capable of absorbing IR radiation from the sun. Because of their symmetry, molecules like N2 and O2 are not.
Visible and ultraviolet (UV)
Light from the visible and UV region of the spectrum is too high to stimulate vibrations of molecules directly, but it is perfect for promoting bound electrons in molecules to higher energy levels. Those transitions from lower to higher energies produce the colors our eyes see. Some of that electronic energy can sometimes be "down-converted" to vibrational energy. If the frequency of UV light is high enough, the extra energy absorbed by bound electrons can cause chemical bonds to break, resulting in damage such as sunburn to human skin.
X-rays and beyond
Light of the energy of x-rays and beyond is often called ionizing radiation. It is capable of breaking chemical bonds and of ejecting the most tightly-bound (core) electrons from atoms and molecules. For biological life, these are damaging energies. On Earth, we are protected from damaging UV radiation by a high atmospheric layer of ozone (O3) molecules, which absorb high-energy UV light efficiently - a sort of global sunscreen.
*
End of digression!
How a greenhouse gets hot
How does a greenhouse get so hot inside? It seems to magnify the heat of the sun, but what it really does is trap some of the heat inside, an ongoing process that builds up over continued exposure to the sun. A sealed greenhouse in the sun (like a sealed car) will get quite hot.
Here's a simplified version of a greenhouse. It's a box (gray) with a single glass wall (blue) on the top. If we let a beam of infrared (IR) light hit the window, we can trace what happens to it. We'll assume that our glass allows about 75% of the light hitting it to pass through, and the other 25% reflects from the surface. That's about average for window glass and IR light.
When light passes through a window, it can either transmit (pass through), reflect (bounce off of a surface) or be absorbed. the latter causes heating of the glass, and is small, so we'll ignore it here.

You've seen this transmission-reflection thing before because you've looked through a window while simultaneously seeing reflections from it.
Start with the red ray on the upper left, labeled with an arbitrary intensity (The amount of energy it carries) of 1. The thickness of the arrow in our model is proportional to light beam intensity.
75% of the original beam of light enters the window and 25% is reflected away, never to return.
Now let's trace that transmitted 75% beam as it reflects from the wall of the greenhouse (bottom of the figure) and hits the window again. 75% of that beam passes back out through the window, but 25% of it reflects off of the window and back into the inside. That's the beam labeled 0.19, which is 25% of 75%. This process continues, with some of the light leaving and some remaining in the greenhouse on each successive encounter with the window.
Continuous radiation by the sun ensures that heat will continue to build up inside the greenhouse in this way, accumulating bit-by-bit as long as the sun is shining. It's that little bit of reflected light that remains in the greenhouse to heat it up.
It's a little more complicated, of course. First, a lot of visible light comes through the glass, too (that's why we see through glass). But this is actually effectively converted (we would say down-converted) to IR light by the plants and objects in the greenhouse, which heat up and re-radiate more IR into the house. Some of that leaves through the window (75% each time it passes) and some is reflected back in, and so on.
A mathematical model
Just for fun, the amount of light remaining in the greenhouse from any one beam of light can be modeled as a geometric series. For any beam that initially gets through, 25% will remain inside and 75% will escape. Our series is
$$I = 0.75 \sum_{n = 0}^{\infty} \left( \frac{1}{4} \right)^n.$$
In our equation, the ¼ is the 25% of each successive beam that stays trapped in the greenhouse.
Heat is trapped by glass because only a portion of the infrared (IR) light that hits it passes through. Some is reflected and thus effectively "trapped" one side.
Earth is a greenhouse (so is Venus)
OK, so how is Earth a greenhouse — it's not surrounded by glass?
That's true, but Earth is surrounded by relatively thin atmosphere (take a look at pictures from space; it really is thin) made up of small gases like nitrogen (N2, oxygen (O2), water (H2O), carbon dioxide (CO2), and others. Of these some are capable of absorbing and re-emitting IR radiation.

When an atmospheric gas molecule absorbs light from the sun, it will eventually re-radiate that light back away from itself. That happens, for example, when a rapidly vibrating molecule bumps into another, and "relaxes" its vibration. The principle of conservation of energy tells us that it must release that extra energy as a "photon" of IR light.
While the light comes from the direction of the sun, The direction of that re-emission is random, so there's about a 50% chance that it will be radiated back toward Earth (because of the geometry, it's actually a little less than 50%). That's alot like a window that passes 50% of the light and reflects 50%, just with a built-in (but very short) delay. So in that way the atmosphere acts just like a window to light.
In our atmosphere, the big IR absorbers are CO2, H2O and CH4. These are heat trapping gases. They regulate the temperature of our planet. Since there has been life on Earth, this semi-transparent window has trapped just enough of the heat from the sun to support a vastly complex web of interconnected living things.

Venus - what happened?
It's worth considering our neighbor planet, Venus, for a second. Roughly the size of Earth, Venus is just one orbit closer to the sun, so we'd expected it to be hotter than Earth, of course. But Venus is hotter even than Mercury, the closest planet to the sun. The mean surface temperature on Venus is more than 450˚C, while that of Mercury is about 165˚C.
The difference between Mercury, Venus and Earth is atmosphere. The atmosphere of Venus is more than 95% CO2. It traps heat so efficiently at the surface that it's difficult for us to imagine anything at all living there. Thermophiles, the Earth organisms that live at the hottest temperatures we know, can survive up to about 120˚C. Mercury has no atmosphere, so any solar energy that hits it is readily re-radiated back into space. Earth is in between.
Life on Earth has evolved over millenia under an atmosphere containing just enough carbon dioxide (CO2) to maintain a fairly steady average global temperature.
Why is Earth warming?
Please note that I'm not going to spend any time in this text responding to fringe theories that human-produced CO2 is not leading directly to planetary warming through the greenhouse effect. Science gave us microwave ovens, flat screen TVs, computers, antibiotics and a host of other conveniences. The self-same science is warning us (and has for a long time) about the effects of our dumping CO2 into the atmosphere. Our task is to accept the results and move forward with our understanding and devising solutions.
For the roughly 100,000 years (4 to 5-thousand generations) that human life has existed on Earth, the number of carbon atoms in the air, water, earth and in living things has been balanced and more-or-less steady. The figure shows the carbon cycle, a rough chart of how carbon atoms move throughout the global biome.
Let's begin and end with carbon in the atmosphere, which exists mainly in the form of carbon dioxide (CO2). CO2 accounts for less than 1% of gases in the atmosphere. Plants of all kinds, including phytoplankton in the oceans, remove CO2 from the atmosphere, using visible light energy from the sun to convert it to carbohydrates, like glucose. So all living things, whether directly or indirectly, remove CO2 from the atmosphere.
CO2 is returned directly to the atmosphere through respiration (CO2 exhalation) of oxygen-breathing animals. It can also be returned through decay processes like composting when plenty of oxygen is available. That kind of aerobic decomposition occurs in the humus of forest floors. Anaerobic decomposition tends to produce other gases like methane, CH4.
But not all living things decay completely into gases. Some organic matter becomes buried, and some of that for millions of years. On Earth, ancient lush forests that died long ago were buried and the carbon they had extracted from the atmosphere formed what are now coal deposits. These are found around the world.
Air-breathing animals, which have lived on Earth far longer than humans (life has existed on Earth for about 3.6 billion years), have also died, partially decomposed and have been buried, forming what are now crude oil deposits.
All of this carbon, taken directly or indirectly from the atmosphere, has been sequestered in what we call fossil fuel deposits, which include coal, oil and organic gases often associated with both. These are reservoirs of carbon which, left alone, would sit there undisturbed for millions of years longer.
The history of humans on Earth spans about 300,000 years, the oldest fossil records being that old. About 100,000 years ago modern humans began moving northward from Africa, eventually inhabiting all continents. During that history, the carbon content of the atmosphere (CO2) has been relatively stable, fluctuating between roughly 175 and 275 parts per million (ppm).
All of the organisms present on the planet for that time have evolved together, each species along its own path, but immensely interconnected, maintaining that carbon balance, and sometimes adapting to other geological forces of the planet.
The carbon cycle

Those organisms (including us) evolved to live under the greenhouse "glass" that is our atmosphere, and that regulates the temperature of Earth.
A new source of CO2
Since humans have discovered how to recover fossil fuels and to release the chemical energy they store by burning them, CO2 concentrations in the atmosphere have increased above the background of natural fluctuations. The global carbon balance has been upset by relatively rapid introduction of CO2 from the burning of coal, oil and gas to drive mobility, generate power and heat and cool dwellings. These are known as anthropogenic (human-caused) carbon sources.
Click or tap the carbon-cycle picture above to add anthropogenic sources of CO2
The graph below shows what we know about global atmospheric CO2 concentrations over the past 800,000 years. The oldest data come from probing deep ice sheets, where pockets of atmospheric gases were trapped thousands of years ago and held in pristine condition as further layers of ice were piled on top. You can see that atmospheric CO2 levels fluctuated between about 175 and 275 parts per million (ppm) until the last few decades, when levels began to increase rapidly. In the last decade, that rate has increased further, and in 2018, the CO2 concentration reached a clear 800,000 year high.
CO2 concentration (ppm) for the past 800,000 years

Tracking anthropogenic CO2
Since scientists first began to measure the concentrations of atmospheric gases, the science has grown increasingly sophisticated and more reliable. We now have redundant, high-quality ways of monitoring the concentration of CO2 and other gases in the atmosphere, both in real time and from the past.
One of the first, and most graphically powerful measurements presented to the public was the atmospheric CO2 measurement at the Mauna Loa observatory in Hawaii. It has been performed consistently since 1958, and shows how atmospheric CO2 concentrations have increased over that time. It shows that shows a roughly 50% increase in CO2 concentration since 1958, and an increasing rate of accumulation (steepness of the curve).
The graph also shows the predictable seasonal variations (the bumps) that occur every year. The measurement is taken roughly hourly. If you follow the link to the Scripps Oceanographic Institute site below the figure, you'll find the data, called the Keeling curve, on several scales. You'll see that the data ties nicely into more recent (pre-1960) data from ice cores and other measurements.

My college biology book — an early warning
I took my first college biology course in 1981. We used the text Biology, 3rd Edition, by Helena Curtis. In the caption to her carbon cycle figure, she wrote,
"Since 1850, carbon dioxide concentrations in the atmosphere have been increasing, owing in part to our use of fossil fuels, to our plowing of the soil and to our destruction of forest land, particularly in the tropics. Some environmentalists predict that this increase in the CO2 "blanket" will increase the temperature here on Earth, with a consequential increase in the great deserts of the world. Others, looking on the sunnier side, foresee an increase in photosynthetic activity because of the increased CO2 that will be available to plants and algae. Most, however feel alarmed by the fact that although we do not know the consequences of what we are doing, we keep right on doing it."
Feedback effects
Higher CO2 concentrations warm Earth through the greenhouse effect. Although the effect is small in magnitude, perhaps a degree Celsius or two so far, its effects could possibly be devastating to many biomes, including those in which humans live.
Rising temperatures have ushered in some feedback effects — other phenomena triggered by warming that will likely contribute to further warming. I'll briefly point out a few here.
Methane release
CO2 is but one of the heat-trapping gases in our atmosphere. There are deposits of methane on sea floors and in oil and coal deposits that are now being released due to direct human activity (mining and drilling, livestock growth), or due to natural sources of methane being released. Methane trapped in arctic tundra, once frozen and thus out of the picture, is now being released because tundra is thawing. Methane in oceanic ices is also at risk for thawing and being released into the atmosphere. The problem here is that methane is a much more potent heat trapping gas than CO2, with the capacity to stimulate even more and more rapid warming.
Increased water vapor
The single most abundant greenhouse gas, it might surprise you to know, is water. Gaseous water is a very efficient heat trapping gas that's always been there. It traps more heat than CO2,, in fact. It's why we have humidity, rain and snow. But warmer air holds more water, and more water means more heat trapping, which means a warmer atmosphere, and so on. That's what feedback means.
Decreased albedo
As global temperatures rise, we see less permanent and seasonal ice around the globe. Glaciers melt and scientists have been tracking loss of ice for a long time.
The polar ice caps, as well as the massive ice sheet on Greenland, are melting.
Ice is a terrific infrared reflector. Albedo is the ability of the planet to reflect incoming radiation, like putting white garments over your skin on a warm day. It reflects a great deal of solar radiation back out to space, helping to keep the planet cool. Without it, we're likely to absorb more solar radiation, further heating the planet, melting more ice ... and so on. Feedback.
One possible mitigating factor for the decreased albedo effect could be increased cloud cover as the atmosphere holds more water vapor. Climate scientists have included this in models that have thus far largely underestimated the rate of global warming.
Forest & ocean degradation
Another result of a warming planet is loss of forests due to increased fire likelihood, intentional deforestation, and incompatibility of forests with a changing climate. It takes time for forests to migrate to climates more like their historical niche climate. Forests are a huge carbon sink. They sequester carbon in living matter, thus it isn't available as a heat-trapping gas.
Rising sea temperatures and levels have caused alarming changes in ocean biomes, including dying coral reefs and dead-zones here and there, where little lives. This is particularly alarming because microscopic ocean photosynthesizers and deposition of carbon-based animals on the sea floor, eventually turning to limestone, are a huge carbon sink. Remember that 70% of Earth is oceans.
This is an incomplete list of feedback effects. My goal was just to acquaint you with some of the bigger ones.
How to learn more
I'll add more sources to this list as I can, but for now, here are a few good sources of further information, digestible by a diligent reader without much knowledge of the scientific details.
| 1. | International Panel on Climate Change Reports The IPCC is an international group of scientists operating within the United Nations, whose task is to periodically collect and analyze climate disruption research, and present their findings to policy makers and the public. Read the latest summary. |
| 2. | Books by Bill McKibben, including The End of Nature. The End of Nature was one of the first popular books on climate change, and remains a good and relevant read. McKibben is a terrific science writer, but you should also read some of his other work. Check out Long Distance. |
| 3. | Films: An Inconvenient Truth and An Inconvenient Sequel. The former won an Academy Award for best original documentary film. The essential science and predictions of the original film have largely proven true, if even conservative in some regards. |
Biome
A biome is a community of organisms linked by common patterns of distinctive features like vegetation and climate. Biomes can be things like grasslands, deserts, rain forests, clumps of tundra grasses. We sometimes refer to all life on Earth as the global biome.
Aerobic / anaerobic
Aerobic biochemical processes are ones that occur in the presence of, and use oxygen, O2. Anaerobic processes don't use oxygen. Because of the difference, the two kinds of processes have different energetics, and produce different kinds of products. Respiration is an aerobic process, and fermentation is anaerobic.
Organic
In chemistry and biology an organic molecule or compound is one that contains carbon atoms. All living matter on Earth is carbon-based. There is a whole branch of chemistry called organic chemistry.
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2012-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.
