Improving upon the ideal gas law
The ideal gas law,
$$PV = nRT$$
where $P$ = pressure, $V$ = volume, $n$ = number of moles of gas, $R$ = the molar gas constant, and $T$ = Kelvin temperature), is an extremely useful relationship. For the most part, it is quite accurate and will suffice for most types of calculations.
The ideal gas law is, however, built on a few key assumptions about gases that may not hold for all gases.
The table below lists some of the key assumptions and how they can fail.
Below, we'll consider another gas equation, very much like the ideal gas law, that can account for some of these non-ideal behaviors.
Assumptions used to develop the ideal gas law and how they can fail
| Assumption | Real gas behavior |
|---|---|
| Gas particles have no volume | Gas particles are atoms of finite size; all have measurable volumes. |
| Gas particles are hard spheres | Gas particles are surrounded by electron "clouds," which can be deformed, polarized &c. |
| Collisions between gas particles are elastic. | Gas particles have a finite attraction for one another, making collisions somewhat "sticky." |
| There are no attractive or repulsive forces between gas particles or with the walls of the container. | Intermolecular forces, attractive or repulsive, can be quite strong between certain atoms & molecules |
The van der Waals equation
In about 1873, Johannes Diderik van der Waals devised a new state equation for gases based on some logical assumptions about how real gases behave.
Later efforts used the principles of statistical mechanics to derive the equation. That's beyond the scope of this page, but we can still take a look at the equation and rationalize its parts. Here is the van der Waals equation:
$$\left( P + \frac{an^2}{V^2} \right) (V - nb) = nRT$$
Notice how similar it is to PV = nRT. It's just that the pressure and volume terms have been modified a bit. Here's another look:

van der Waals included two adjustable parameters in his equation, $a$ and $b$, to represent the finite size of gas particles ($b$) and a characteristic "stickiness" or attraction between particles ($a$).
Let's try to unpack the equation a bit. First notice that if both $a$ and $b$ are zero, we're right back to the ideal gas law.
In the first parentheses, the pressure is augmented by a term proportional to parameter $a$ and the square of the number of moles of gas, and inversely proportional to the square of the volume. This reflects the fact that real gases, which have some amount of inter-particle attraction, are more compressible than ideal gases. In small volumes, the term added to the pressure can be relatively large.
In the second parentheses, The volume available to the gas (the volume of the container) is reduced by the finite volume of the gas particles, where $b$ is the volume taken up per particle.
Here is a limited table of van der Waals $a$ and $b$ parameters for some common gases.
| Gas | a (L2·atm·mol-2) | b (L·mol-1) | N2 | 1.39 | 0.0391 |
|---|---|---|
| O2 | 1.382 | 0.03186 | CO2 | 3.658 | 0.04286 |
| H2O | 5.537 | 0.03049 |
| He | 0.0346 | 0.0238 |
| CCl4 | 20.01 | 0.1281 |
| SF6 | 7.857 | 0.08786 |
| Xe | 4.192 | 0.05156 |
Example – water
Here is an example of the difference between ideal gas behavior and more realistic behavior, as modified by the van der Waals equation. The graph shows pressure vs. volume for a mole of water molecules at 300K.
Beyond a volume of about 1 Liter, both the ideal gas law (black curve) and the van der Waals equation give approximately the same result. Namely, for a given volume, the pressure is roughly the same. But as water is further compressed, notice that the pressure decreases dramatically. This is due to the intermolecular attraction for water molecules for each other. Ultimately, that behavior leads to complete condensation into the liquid state, in which few water molecules are able to contribute to pressure on the walls of a container.
You can see from the table of van der Waals constants, that we'd expect even larger effects for carbon tetrachloride (CCl4) and sulfur hexafluoride (SF6).
Pressure vs. volume,
1 mole of H2O at 300K
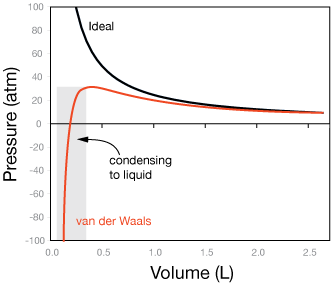
The graph shows that for dilute gases, the ideal gas law isn't bad, even for water, which is quite sticky to itself.
Example – CO2
A 5.0 L container holds 120 g of CO2 gas at 230˚C. Calculate the pressure of the gas
- using the ideal-gas law, and
- using the van der Waals equation.
- Explain the difference between the results.
$$ \require{cancel} \begin{align} PV &= nRT \; \color{magenta}{\longrightarrow} \; P = \frac{nRT}{V} \\[5pt] &= \frac{120 \, \cancel{g} \frac{1 \, \cancel{mol}}{44 \, \cancel{g}} \left( 0.0821 \frac{ \cancel{L} atm}{\cancel{mol} \cancel{K}} \right) (403 \, \cancel{K})}{5.0 \, \cancel{L}} \\[5pt] &= {\bf 18.04 \; atm} \end{align}$$
Now the van der Waals equation:
$$\left( P + \frac{an^2}{V^2} \right) (V - nb) = nRT$$
Rearranging gives
$$P = \frac{nRT}{V - nb} - \frac{an^2}{V^2}$$
Now we'll plug in numbers, n = 2.727 mol from the calculation above, and a & b from the table above:
$$ \begin{align} &= \frac{2.727 \cancel{mol} \left(0.0821 \frac{\cancel{L} atm}{\cancel{mol} \cancel{K}} \right)(403 \, \cancel{K})}{5 \cancel{L} - 2.727 \cancel{mol}\left(0.04286 \frac{\cancel{L}}{\cancel{mol}} \right)} \\[5pt] &\phantom{0000} - \frac{3.658 \frac{\cancel{L^2} atm}{\cancel{mol^2}} (2.727^2 \cancel{mol^2})}{5.0^2 \cancel{L^2}} \\[5pt] &= {\bf 17.39 \, atm} \end{align}$$
That's about a 3.6% difference from the ideal-gas expectation. It might be enough to change the outcome of some experiments. The difference is due to the existence of attractive intermolecular forces between CO2 molecules, and the fact that they have nonzero volume.
Practice problems
-
Calculate the pressure of 10 g of CCl4 (carbon tetrachloride) contained in a 200 mL volume at 20˚C using both the ideal gas law and the van der Waals equation.
Solution
First, 10 grams of CCl4 is
$$10 \cancel{g} \left( \frac{1 \, mol}{153.82 \cancel{g}} \right) = 0.065 \, mol$$
The ideal gas equation gives
$$PV = nRT \; \color{magenta}{\longrightarrow} \; P = \frac{nRT}{V}$$
$$ \begin{align} &= \frac{0.065 \cancel{mol} \left( 0.0821 \frac{ \cancel{L} atm}{\cancel{mol} \cancel{K}} \right) (293 \, \cancel{K})}{0.2 \, \cancel{L}} \\[5pt] &= {\bf 7.82 \; atm} \end{align}$$
The van der Waals equation,
$$P = \frac{nRT}{V - nb} - \frac{an^2}{V^2}$$
(omitting units for clarity, and taking the a and b values from the table above, yields
$$ \begin{align} &= \frac{0.065 (0.0821)(293)}{0.2 - 0.065(0.1281)} \\[5pt] &\phantom{0000} - \frac{20.01 (0.065^2)}{0.2^2} \\[5pt] &= {\bf 6.04 \, atm} \end{align}$$
-
5.0 moles of N2 gas are contained in a volume of 0.40 L. The container is heated to 125˚C. Calculate the temperature of the N2 gas using both the ideal gas law and the van der Waals equation.
Solution
The ideal gas equation gives
$$PV = nRT \; \color{magenta}{\longrightarrow} \; P = \frac{nRT}{V}$$
$$ \begin{align} &= \frac{5 \cancel{mol} \left( 0.0821 \frac{ \cancel{L} atm}{\cancel{mol} \cancel{K}} \right) (398 \, \cancel{K})}{0.4 \, \cancel{L}} \\[5pt] &= {\bf 408 \; atm} \end{align}$$
The van der Waals equation,
$$P = \frac{nRT}{V - nb} - \frac{an^2}{V^2}$$
(omitting units for clarity, and taking the a and b values from the table above, yields
$$ \begin{align} &= \frac{5.0 (0.0821)(398)}{0.4 - 5.0(0.0391)} \\[5pt] &\phantom{0000} - \frac{1.39 (5.0^2)}{0.4^2} \\[5pt] &= {\bf 582 \, atm} \end{align}$$
-
How many moles of sulfur hexafluoride (SF6) are needed to achieve a pressure of 3.00 atm in a volume of 1 L at room temperature, 25˚C ? Use both the ideal gas law and the van der Waals equation and compare the solutions.
Solution
The ideal gas equation gives
$$PV = nRT \; \color{magenta}{\longrightarrow} \; n = \frac{PV}{RT}$$
$$ \begin{align} &= \frac{3 \cancel{atm} (1 \, \cancel{L})}{\left( 0.0821 \frac{ \cancel{L} \cancel{atm}}{mol \cancel{K}} \right) (298 \, \cancel{K})}\\[5pt] &= {\bf 0.123 \; mol} \end{align}$$
To use the van der Waals equation to solve for the number of moles, it's straightforward just to plot
$$\left( P + \frac{an^2}{V^2} \right)(V - nb) \: \: \text{and} \: \: nRT$$
versus n, and locate the intersection of those two curves. Using SF6 data from the table, we obtain the graph

So the number of moles, $n = {\bf 0.128 \, mol},$ is slightly larger using the non-ideal case and data.
Parameter
A parameter is an adjustable constant in the definition of a function that is different from the independent variable(s). Parameters are not independent variables. For example, in the quadratic function
f(x) = Ax2 + Bx + C
A, B and C are parameters which change the shape of the graph of the function. x is the independent variable. A, B and C are fixed for any particular version of f(x), but x can range from -&inf; to +&inf;

![]()
xaktly.com by Dr. Jeff Cruzan is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. © 2016-2025, Jeff Cruzan. All text and images on this website not specifically attributed to another source were created by me and I reserve all rights as to their use. Any opinions expressed on this website are entirely mine, and do not necessarily reflect the views of any of my employers. Please feel free to send any questions or comments to jeff.cruzan@verizon.net.